
Torino Phytoplasma – Host Interaction Group
Institute for Sustainable Plant Protection – National Research Council of Italy
Team description
Relationships among phytoplasmas and their plant and insect hosts are our main interest. Both fundamental and applied studies are pursued with the aim of developing effective and sustainable tools/strategies to control phytoplasma diseases. Model and field patho-systems are investigated using bio-assays, molecular biology, bioinformatics, and microscopy to address plant and insect responses to phytoplasma infections.

Phytoplasma patho-systems
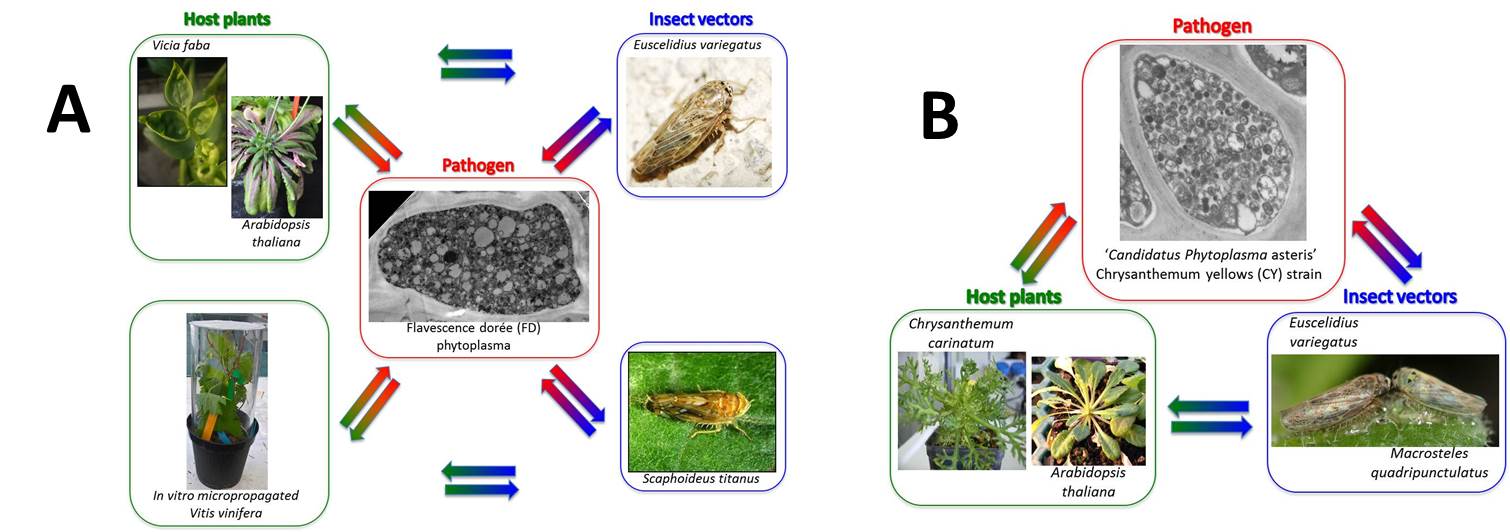
Flavescence dorée (FD) is the main problem of viticulture in Southern Europe and the phytoplasma that causes it, is included in the European list of quarantine organisms. The disease is a real threat to the Piedmont wine-growing areas. At the moment there is no genetic resistance to FD since all the cultivars show different degrees of susceptibility to the disease. The FD phytoplasma is a wall-less bacterium belonging to the 16S ribosomal subgroups V-C and –D, with three main genetic clusters based on sequences from other genes. Two strains are routinely maintained in our greenhouses: FD-Piedmont (V-C), and FD-AT (V-D). The phytoplasma is transmitted to grapevine mainly by Scaphoideus titanus, although other leafhopper species may transmit this pathogen either to grapevine or to other herbaceous hosts, such as broad bean. Under laboratory conditions, FD phytoplasma (FDp) is transmitted to broadbean and Arabidopsis thaliana by infective Euscelidius variegatus and to grapevine plantlets issued from in vitro propagation by infective S. titanus hatched from winter-collected grapevine wood (Fig. A).
Chrysanthemum yellows (CY) is a disease of ornamental plants, originally isolated in the Italian Riviera. It is transmitted by Macrosteles quadripunctulatus, E. variegatus and Euscelis incisus. CY is caused by ‘Candidatus Phytoplasma asteris’ (CYp). Under laboratory conditions, CYp phytoplasma is transmitted to Chrysanthemum carinatum and A. thaliana by infective M. quadripunctulatus or E. variegatus (Fig. B).
Phytoplasma – plant interactions
The main goals of our group are:
- to identify the plant mechanisms in response to phytoplasma infection by integration of genetic data obtained through Next Generation Sequencing techniques, and validation of the biological role of the identified genes, using thaliana mutants in a ‘reverse genetics’ approach. This will provide the indispensable material to identify the target genes necessary for the creation of resistant / FD tolerant grapevine genotypes, which can be integrated into a sustainable strategy to contain phytoplasma infections.
- to characterize FD disease in the context of the local viticulture, by modeling the FD epidemics and by characterizing and quantifying the susceptibility to FDp of various Vitis vinifera varieties, with special regard to local cultivars seriously endangered by the disease.
- to investigate generic and specific phytoplasma infection mechanisms, by exploiting the model FD and CY patho-systems. Events at the specific tissues of plant host and insect vector tissue level involved in the infection process are explored through integration of different innovative technologies, such as RNA Deep Sequencing (RNA-seq), Laser Microdissection (LMD) and digital PCR (dPCR), to describe the infection process lead by two phylogenetically different phytoplasmas studied in vivo, at single host cell level, in the same plant species and insect vector. Phytoplasma transcriptomics in different hosts and experimental conditions will also be addressed.
Further reading
Chitarra W., Pagliarani C., Abbà S., Boccacci P., Birello G., Rossi M., Palmano S., Marzachì C., Perrone I., Gambino G. miRVIT: a novel miRNA database and application to uncover Vitis responses to Flavescence dorée infection. 2018. Frontiers in Plant Science, 9:1034. doi: 10.3389/fpls.2018.01034
Maggi F., Bosco D., Galetto L., Palmano S., Marzachì C. Space-time point pattern analyses of Flavescence dorée epidemic in a grapevine field: disease progression and recovery. 2017. Frontiers in Plant Science, 7: 1987-1998. doi: 10.3389/fpls.2016.01987
Pacifico, D., L. Galetto, M. Rashidi, S. Abba, S. Palmano, G. Firrao, D. Bosco, C. Marzachì. 2015. Decreasing global transcript levels over time suggest that phytoplasma cells enter stationary phase during plant and insect colonization. Applied and Environmental Microbiology, 81(7): 2591-2602. doi 10.1128/AEM.03096-14
Abbà S., Galetto L., Carle P., Carrère S., Delledonne M., Foissac X., Palmano S., Veratti F., Marzachì C. 2014. RNA-Seq profile of Flavescence dorée phytoplasma in grapevine. BMC Genomics, 15(1):1088. doi:10.1186/1471-2164-15-1088
Phytoplasma – insect interactions
The main goals are:
- to identify molecular interactions between partners involved in phytoplasma transmission specificity by in vitro and in vivo RNA interference (RNAi) of putatively interacting genes is applied for further validation and functional genetics studies. Also, NGS is used to identify insect genes altered upon phytoplasma infection, and cellular, enzymatic, biological experiments are used to validate corresponding phenotypes.
- to describe transmission characteristics of phytoplasmas in single or multiple infections.
- to search for insect viruses as potential biocontrol tool to suppress vector population or as vectors of gene silencing to interfere with the expression of vector genes putatively involved in the transmission process, as well as selected phytoplasma effectors
Further reading
Galetto L., Abbà S., Rossi M., Vallino M., Pesando M., Arricau-Bouvery N., Dubrana M.P., Chitarra W., Pegoraro M., Bosco D. Marzachì C. 2018. Two phytoplasmas elicit different responses in the insect vector Euscelidius variegatus Kirschbaum. Infection and Immunity, 86:e00042-18, doi: 10.1128/IAI.00042-18
Abbà S., Galetto L. , Vallino M. , Rossi M. , Turina M., Sicard A., Marzachì C. 2017. Genome sequence, prevalence and quantification of the first iflavirus identified in a phytoplasma insect vector. Archives of Virology, 162:799–809. doi:10.1007/s00705-016-3158-3
Rashidi M., Galetto L., Bosco D., Bulgarelli A., Vallino M., Veratti F., Marzachì C. 2015. Role of the major antigenic membrane protein in phytoplasma transmission by two insect vector species. BMC Microbiology.2015, 15:193, doi: 10.1186/s12866-015-0522-5
Rashidi M., D’Amelio R., Galetto L., Marzachì C., Bosco D. 2014. Interactive transmission of two phytoplasmas by the vector insect. Annals of Applied Biology, 165(3): 404-413. doi:10.1111/aab.12146
Galetto L., Bosco D., Marzachì C. 2013. Selection of reference genes from two leafhopper species challenged by phytoplasma infection, for gene expression studies by RT-qPCR. BMC Research Notes, 6(1):409. doi: 10.1186/1756-0500-6-409
Galetto L., Bosco D., Balestrini R., Genre A., Fletcher J., Marzachi’ C. 2011. The Major Antigenic Membrane Protein of ‘‘Candidatus Phytoplasma asteris’’ Selectively Interacts with ATP Synthase and Actin of Leafhopper Vectors. PLOS ONE 6(7): e22571 doi: 10.1371/journal.pone.0022571
FD epidemiology
The main goal is to characterize Flavescence dorée disease taking into account plant varieties, phytoplasma genetic diversity, phytoplasma multiplication and gene regulation in the two hosts (plant and vector), insect fitness and feeding behavior in relation to different grapevine genotypes. We aim at implementing this knowledge to estimate transmission efficiency under different plant genotype/phytoplasma strain/insect vector combinations.
Further reading
Maggi F., Bosco D., Galetto L., Palmano S., Marzachì C. Space-time point pattern analyses of Flavescence dorée epidemic in a grapevine field: disease progression and recovery. 2017. Frontiers in Plant Science, 7: 1987-1998. doi: 10.3389/fpls.2016.01987
Galetto L., Miliordos D. E., Pegoraro M., Sacco D., Veratti F., Marzachì C., Bosco D. 2016. Acquisition of Flavescence dorée phytoplasma by Scaphoideus titanus Ball from different grapevine varieties. International Journal of Molecular Sciences, 17(9), 1563. doi:10.3390/ijms17091563. ISSN: 1422-0067
Galetto L., Miliordos D., Roggia C., Rashidi M., Sacco D., Marzachì C., Bosco D. 2014. Acquisition capability of the grapevine Flavescence dorée by the leafhopper vector Scaphoideus titanus Ball correlates with phytoplasma titre in the source plant. Journal of Pest Science, 87(4), 87:671-679. doi 10.1007/s10340-014-0593-3
Group Leader
Permanent lab members
Flavio Veratti
PhD students, post docs and contract staff
Federica Tota
